hcooch ch2 h2o
Industrial Synthesis: Carbonylation of Methanol
The primary industrial method for producing methyl formate involves the carbonylation of methanol (). This process reacts methanol with carbon monoxide () under high pressure and temperature, often using a sodium methoxide hcooch ch2 h2o catalyst:
This process is highly efficient and is the dominant route for large-scale production, ensuring a high-purity product essential for its use as a solvent in the electronics and fine chemical industries.
Physical and Chemical Properties
Methyl formate is characterized by its low boiling point (approximately or ), making it highly volatile and contributing to its utility as a fast-evaporating solvent. It is moderately soluble in water and miscible with most organic solvents. Importantly, its volatility poses fire risks, and its vapors can be irritating. The molecule’s polarity allows it to dissolve a wide range of organic substances, making it suitable for applications such as a blowing agent for certain foam polymers and as a carrier solvent for lacquers.
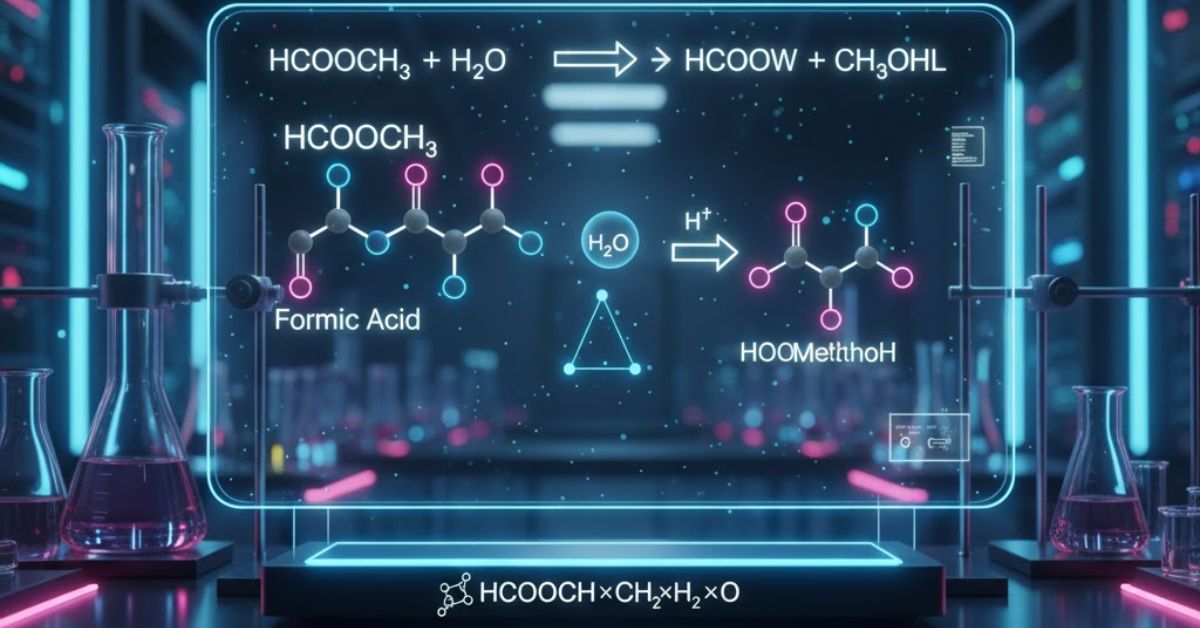
The Hydrolysis Reaction ()
The core reaction suggested by the keyword is the hydrolysis of methyl formate. This is the reverse of the esterification reaction, where the ester reacts with water (hcooch ch2 h2o) to form its parent acid and alcohol. The reaction is typically catalyzed by an acid (like ) or a base (like ):
This reaction is crucial in chemical processing because it demonstrates the fundamental principle of chemical equilibrium and is used in industrial settings to regenerate formic acid or methanol from waste streams of the ester.
Mechanistic Analysis of Acid-Catalyzed Hydrolysis
The acid-catalyzed hydrolysis of methyl formate follows a specific mechanism known as (Acid-catalyzed, Acyl-oxygen cleavage, Bimolecular). In this mechanism:
-
The carbonyl oxygen of the ester is first protonated by the acid catalyst ().
-
A molecule of water then acts as a nucleophile, attacking the electron-deficient carbonyl carbon.
-
A tetrahedral intermediate is formed.
-
Subsequent proton transfers and the departure of the alcohol () yield the final products, formic acid and the regenerated acid catalyst.
This precise mechanism illustrates why the reaction is reversible and is crucial for controlling the synthesis or decomposition of the ester.
The Role of Formic Acid ()
The product of the hydrolysis, formic acid (hcooch ch2 h2o), is a key industrial chemical used in livestock feed preservation, the textile industry, and leather tanning. The ease with which it can be produced via the hydrolysis of methyl formate makes the ester a viable and often safer precursor compared to handling concentrated formic acid directly, which is corrosive and hazardous.
Environmental and Safety Considerations
Methyl formate’s volatility and relatively low flash point necessitate stringent safety protocols in its handling and storage. It is classified as an extremely flammable liquid. From an environmental perspective, while it is biodegradable, the large-scale use of its products requires careful management. The hydrolysis reaction is sometimes relevant in the environmental fate of the compound, determining its persistence in aqueous systems.
Application as a Refrigerant
In a modern, less common application, methyl formate has been investigated for use as a refrigerant (). Its thermodynamic properties make it a viable alternative in certain industrial refrigeration cycles. This application is significant as industries search for refrigerants with a lower Global Warming Potential (GWP) compared to traditional hydrofluorocarbons (hcooch ch2 h2o), aligning its use with increasing environmental regulations.
The Role of the Component in the Formula
The inclusion of the term in the user’s initial query () might imply an intended formula for ethyl formate (hcooch ch2 h2o) which would have an additional group, undergoing the exact same hydrolysis reaction to yield formic acid and ethanol (). Alternatively, it could incorrectly reference an intermediate step, such as the formation of formaldehyde () as a product of methanol degradation, but the most chemically sound interpretation remains the hydrolysis reaction.
Conclusion: A Foundation of Organic Chemistry
Methyl formate, as suggested by the core formula hcooch ch2 h2o and its reaction with water , stands as a foundational molecule in organic chemistry and industrial practice. Its simple structure provides a perfect model for studying ester hydrolysis, a reaction that is central to industrial synthesis, biological processes (like the breakdown of fats), and the environmental cycling of organic compounds. The chemistry surrounding this molecule highlights the critical interplay between synthesis, equilibrium, and industrial application.



